How to Read Temperature and Pressure Charts of Gases
Gay-Lussac's Law Temperature-Pressure Human relationship in Gases and the Conclusion of Absolute Zip
By Chuck Roser, Retired Chemical science Instructor
N Carolina School of Science and Mathematics
Next Generation Science Standards
| Science and Engineering Practices | Disciplinary Core Ideas | Crosscutting Concepts |
| Practice: Planning and conveying out investigations Students will investigate the relationship betwixt temperature and pressure of a closed container. | DCI: Matter and its interactions • Students will decide the relationship between pressure and temperature and chronicle temperature to kinetic free energy and motion of particles. | Concept: Scale, proportion, and quantity • Students will establish a proportional human relationship between temperature and force per unit area. |
Objectives
This experiment's objectives are to (a) observe the human relationship between the pressure and temperature of a abiding number of moles of gas in a abiding book container, and (b) to experimentally determine an estimated value for accented zero.
Introduction
When a gas is heated, its molecules' average speed and kinetic energy are increased. If the container has a abiding volume, the molecules will strike its sides with greater frequency. This creates greater strength on the container'southward walls per unit area, increasing pressure level in the container. Information technology is one of the bases for the warning confronting heating an droplets spray can.
A graph may be plotted to show how the force per unit area of a fixed mass of gas varies as the temperature is inverse. The temperature at which the pressure of an platonic gas would, in theory, reach zero can be determined by extrapolating the pressure vs. temperature graph to cipher pressure. This temperature is referred to every bit absolute aught and is the nil point for the Kelvin temperature scale. An extrapolation to zero pressure is necessary because real gases condense to liquids and solidify before reaching absolute zero. The relationship betwixt the pressure of an ideal gas and its Kelvin temperature is expressed in Gay-Lussac's police:
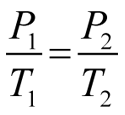
In this experiment, the pressure within the Absolute Zero Demonstrator apparatus is measured at several dissimilar temperatures. A graph of pressure vs. temperature is then prepared to institute the relationship between pressure level and temperature and to gauge a value for absolute nix. The apparatus consists of a copper bulb having a stock-still volume (copper expands and contracts but slightly with temperature), a force per unit area gauge, and a fixed mass of gas. Gas pressure is measured with the pressure gauge. Before a measurement is taken, the apparatus is immune to equilibrate to ensure that the gas and bulb are at the aforementioned temperature.
- 2 2-L Beakers (for boiling-water baths)
- ii Stirring Hot Plates (with big magnetic stir bars)
- 2 Celsius Thermometers (alcohol thermometers are preferable)
- 2 Ring Stands (with large 3-prong clamps and clench holders to hold the Absolute Zippo Demonstrator)
- two Small 3-Prong Clamps and Clamp Holders (to hold the thermometers)
- four 2-L Beakers (for the 50/50 humid water/room temperature water bath; the room temperature water bath; the ice water bathroom; and the dry ice-ethanol, or acetone, bathroom)
- Absolute Zero Demonstrator
- i Pair Insulated Thermal Gloves (for handling the water baths, dry ice, liquid nitrogen, and Absolute Null Demonstrator)
- Wide-Mouth Dewar Flask (with liquid nitrogen) (optional)
Safety
- Caution: Do not employ open flames during the experiment. Ethanol and acetone are very flammable.
- Caution: Dry ice and liquid nitrogen should exist handled very carefully (wear safety glasses and insulated thermal gloves) due to the risk of frostbite.
- Circumspection: Never put dry water ice or liquid nitrogen in a closed container because each volition build up pressure and explode the container.
- Wear safety glasses during the experiment.
- Use insulated thermal gloves and appropriate care when handling the water baths, dry ice, liquid nitrogen, and Accented Zero Demonstrator.
Procedure
- All participants put on safety glasses. Individuals responsible for handling the Accented Zero Demonstrator appliance, water baths, dry water ice, and liquid nitrogen put on insulated thermal gloves.
- Immerse the appliance's bulb in the boiling-water bath. Support the appliance with a large 3-prong clamp and clamp holder and support the thermometer with a small three-prong clamp and clamp holder. Allow the water to return to a full boil.
- Wait a few minutes for the apparatus to equilibrate. The pressure reading should stabilize at a constant value.
- Tape the force per unit area to the nearest mm Hg and temperature to the nearest 0.i° C.
- Remove the apparatus from the bath.
- Carefully pour out near 1-half of the boiling water and replace it with room temperature water to create a 50/l boiling-water/room temperature water bathroom. Immerse the apparatus's seedling in the bath. Support the apparatus and thermometer equally described in step 2. Repeat steps 3 to 5.
- Immerse the apparatus'southward bulb in the room temperature water bath. Support the apparatus and thermometer as described in step 2. Repeat steps iii to five.
- Immerse the apparatus's seedling in the ice h2o bath. Back up the apparatus and thermometer as described in step 2. Repeat steps iii to five.
- Immerse the apparatus'south bulb in the dry out ice-ethanol (or acetone) bathroom. Support the apparatus as described in step 2. Practise non utilise a thermometer to measure the temperature. The dry ice bath is assumed to be at the sublimation temperature of carbon dioxide, 1 atm force per unit area and –78.v° C. Expect a few minutes for the apparatus to equilibrate. Record the pressure guess reading and –78.v° C.
- Remove the appliance from the bath.
- Optional: Purge the apparatus with helium. Immerse the apparatus'due south seedling in a wide-oral cavity Dewar flask containing liquid nitrogen. Back up the appliance every bit described in step 2. Exercise not use a thermometer to mensurate the temperature. The liquid nitrogen bath is assumed to exist at its boiling point, one atm pressure and –195.7° C. Wait a few minutes for the apparatus to equilibrate. Record the force per unit area gauge reading and –195.7° C.
- Remove the apparatus from the bath.
Information analysis
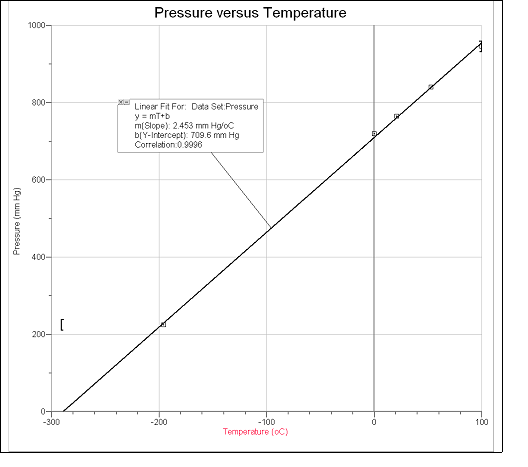
- Set a graph of pressure level vs. temperature in °C. This graph should have labeled axes with units as follows: scale the x-axis from –300 to 100° C (or a value negative enough to clearly bear witness the x-intercept) and the y-axis from 0 to
i,000 mm Hg. Draw the best direct line through the data. Extend the line until it intersects the x-axis. The x-intercept (pressure = 0) gives the extrapolated value for absolute zero. - If you lot take access to a graphing calculator or graphing software, graph the information and perform a linear regression analysis.
- Record the slope, y-intercept, and correlation coefficient (r). The closer the value of r is to 1.00 or –1.00, the better the information fit a straight line.
- Follow your teacher's directions about printing the graph with the linear regression line and regression statistics.
- To solve for the value of absolute zilch, use the equation for a line, y = mx + b . Accented nix is the temperature at which the gas's pressure equals null. This is the line's 10-intercept. To calculate this value, set up y = 0, substitute in the value of the slope, and solve for ten.
Information table
| Reading No. | Type of Bath Used | Temperature (°C) | Temperature (°One thousand) | Pressure (mm Hg) |
| ane | ||||
| 2 | ||||
| 3 | ||||
| 4 | ||||
| five | ||||
| 6 |
Attach a graph of pressure vs. temperature in °C. This graph should have labeled axes with units as follows: scale the 10-axis from –300 to 100° C (or a value negative enough to conspicuously show the x-intercept) and the y-axis from 0 to 1,000 mm Hg. Evidence the linear regression line through your data with the slope, y-intercept, and correlation gene.
Calculations and questions
- Regression analysis:
Linear regression statistics: slope: _________ y-intercept: _______ correlation factor (r): _____.
Equation of the regression line:
Use the slope and a y value of nil to calculate a value for absolute zero:___________. Show your calculations.
- Calculate your relative percent mistake for the value of absolute zero based on an accepted value of –273° C.

- Are pressure and temperature straight or inversely related? Justify your answer based on your graph.
- Talk over any sources of mistake that occurred or any improvements that could exist made in this experiment.
Instructor's notes
- Caution: Do not employ open flames during the experiment. Ethanol and acetone are very flammable.
- Caution: Dry out ice and liquid nitrogen should be handled very carefully (wearable safety glasses and insulated thermal gloves) due to the risk of frostbite.
- Caution: Never put dry out ice or liquid nitrogen in a closed container because each volition build upward pressure and explode the container.
- Sample information:
Reading No. Type of Bathroom Used Temperature (°C) Temperature (°K) Pressure (mm Hg) 1 Boiling h2o 100.0 373.one 945 two Boiling water + room temp. h2o 53.0 326.one 840 3 Room temp. water 21.0 294.i 765 four Ice h2o 0.0 273.i 720 five Dry ice/ethanol (or acetone) –78.5 6 Liquid nitrogen –195.7 77.4 225 - It is easier to have 1 beaker available for each bath. Accept the boiling-water, room temperature h2o, water ice water, and dry ice/acetone baths already prepared. Have an actress-large chalice of boiling water available for making the humid-water/room temperature water bath. Bank check the guess amount of water needed to cover the seedling in the bath. Notation: Be careful non to overfill the beakers or they volition overflow when the seedling is immersed.
- Different pairs of students tin read the pressure and temperature values for each data point. Note: A mercury thermometer cannot be used with the dry ice/acetone or liquid nitrogen baths since mercury freezes at –39° C. An booze thermometer will freeze in liquid nitrogen. The dry ice/acetone bath is assumed to be at the sublimation temperature of 1 atm and –78.v° C, and the liquid nitrogen at its boiling point of 1 atm and –195.seven° C.
- A linear human relationship between pressure and temperature tin can be demonstrated with the iv h2o bath temperatures. A good value for absolute zero requires a low-temperature data betoken to reduce the range over which the extrapolation is done. The dry water ice/acetone bathroom provides a skilful low-temperature data bespeak; notwithstanding, the liquid nitrogen value works better since it is closer to accented zip.
- The device should be purged with helium if liquid nitrogen is used since the oxygen in air will condense at the temperature of liquid nitrogen, causing the pressure reading to be too low.
- Denatured ethanol and acetone can be obtained from a hardware store. Dry ice can be obtained from grocery or political party stores. A Dewar flask and liquid nitrogen tin sometimes exist obtained from a local college.
- The dry ice is allowed to sublime at the end of the lab and the ethanol or acetone can be reused. The liquid nitrogen is allowed to evaporate.
Source: https://www.carolina.com/teacher-resources/Interactive/gay-lussacs-law-temperature-pressure-relationship-in-gases-determination-absolute-zero/tr10730.tr
Post a Comment for "How to Read Temperature and Pressure Charts of Gases"